Abstract
INTRODUCTION: Intensive care unit (ICU) admission and multi-organ support is fundamental in a subset of patients with hematologic malignancies and severe illness, guaranteeing the opportunity to accomplish a therapeutic plan, frequently with curative intent. Nevertheless, given the high associated mortality and absence of specific outcome predictors, those who truly benefit from multiple organ support are still largely unknown a priori. There is also an urgent need to attain better outcomes in this specific subset of patients.
OBJECTIVES: Identification of outcome predictors in patients with hematologic malignancy and multisystemic organ failure admitted to an ICU. Mortality and functional outcome 1-month and 1-year after ICU discharge were the primary and secondary outcomes.
METHODS: Retrospective, single centre study from a tertiary care, university hospital. Clinical and laboratory data from patients with hematologic malignancies admitted to ICU (June 2014 to December 2021) were extracted from electronic health records. Functional assessment was performed through clinical registries plus phone contact and scored according to Eastern Cooperative Oncology Group (ECOG) scale. Statistical analysis was performed using IBM® SPSS® Statistics 25.
RESULTS: We included 147 patients, 61.9% male with a median age of 60 years old, median SAPS II of 60. Acute leukemia (36.1%), non-Hodgkin lymphoma (34.7%) and multiple myeloma (9.5%) were the most prevalent diagnosis. Median length of ICU stay was 3 days (IQR: 1-7). Most patients had active disease (70%) and were under treatment on ICU admission, 50% on first line and 30% on second line therapy. Allogeneic stem cell transplant recipients represented 17% of our sample. Septic shock (40.1%) and respiratory failure (38.8%) were the most frequent causes for ICU admission. During the ICU stay there was a median of 3 organ failures. Cardiovascular failure was present in 68.8% of patients and respiratory failure in 81%, with 54% receiving invasive mechanical ventilation.
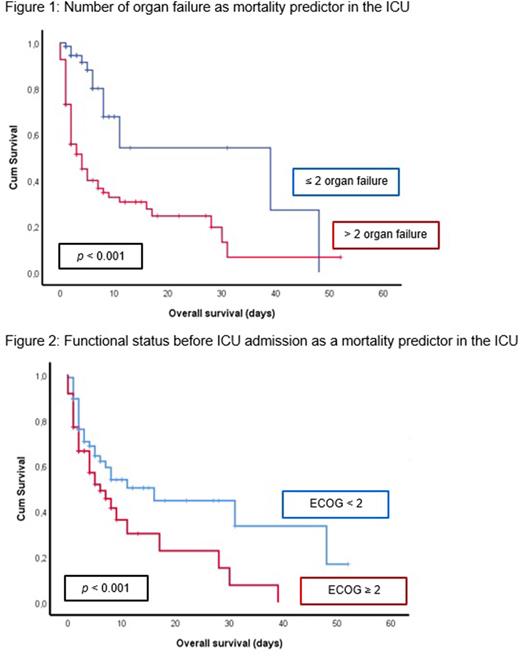
We observed a ICU mortality of 45.5%. Main cause of death was refractory septic shock (60.9%), followed by acute toxicity secondary to chemotherapy (16.4%) and progressive disease (10.4%). Cumulative organ failure was one of the main mortality predictors: patients with >2 organ failure had 35% higher probability of mortality (HR 3.88, 95% IC 2.1 - 7.3, p < 0.001; figure 1). On multivariate analysis, presence of either respiratory, cardiovascular and liver dysfunction were significant mortality predictors. Functional status preceding ICU admission was also a significant predictor of mortality: patients with ECOG ≥2 had significantly lower ICU survival rates (p=0.01; figure 2). One month after ICU discharge, ECOG ≥2 was associated with an 47% increase in probability of death (HR 4.33, 95% IC 2.0 - 9.3, p < 0.001).
Age, active disease, hematologic diagnosis, number of lines of therapy, allogeneic stem cell transplant and length of organ support weren't significant predictors of mortality.
Among those who survived to ICU discharge, 47% are alive after 1 year of follow up and 63% continued treatment and achieved a complete response. Their functional status improved, with 42% with ECOG <2.
CONCLUSIONS: Fewer organ disfunctions and better functional status before ICU admission helps to identify those who strongly benefit from ICU care. Early admission to ICU, by limiting cumulative organ disfunction, one of the strongest mortality predictors, may contribute to a better prognosis. Poor functional status before ICU admission also presented as a strong mortality predictor. Those who have a better functional status 1 month after ICU discharge have a lower probability of mortality, highlighting the importance of functional status recovery after ICU care. Importantly, most patients who are alive one year after ICU admission are in complete hematologic response.
Disclosures
Alves:AbbVie: Consultancy, Other: Lecture Fees; Takeda: Consultancy, Other: Lecture Fees; AstraZeneca: Consultancy, Other: Lecture Fees; Roche: Consultancy, Other: Lecture Fees; Janssen-Cilag: Other: Lecture Fees; Gilead: Other: Lecture Fees; EUSA Pharma: Other: Lecture Fees. Lacerda:Abbvie: Consultancy; Pfizer: Consultancy; Merck Sharp & Dohme: Consultancy; Amgen: Consultancy; Gilead Sciences: Consultancy; Takeda: Consultancy. Raposo:Takeda: Consultancy; Janssen: Consultancy; Gilead Sciences: Consultancy; Abbvie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal